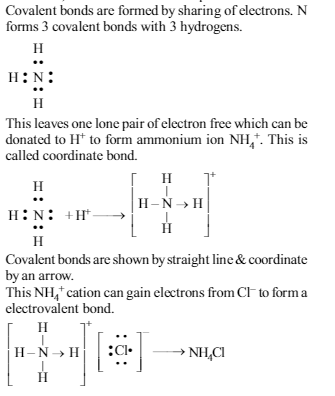
In which of the following compound electrovalent, covalent and co-ordinate bonds are present?
-
Solution

For AB bond if percent ionic character is plotted against electronegativity difference (XA– XB), the shape of the curve would look like

-
Solution
Percent ionic character is given by following equation.% of ionic character = 16(XA– XB) + 3.5(XA – XB)2 From the above relation, it is clear that as soon as(XA– XB) increases, % ionic character will also increase.Therefore,curve C show a correct path.
Which does not show resonance :
-
Solution
Benzene and the compounds having benzene ring show resonance. Hence, ethylamine does not show resonance.
Polarisibility of halide ions increases in the order
-
Solution
In case of anions having same charge as the size of anion increases, polarisibility of anion also increases.
Which bond angle θ would result in the maximum dipole moment for the triatomic molecule YXY
-
Solution

As compared to covalent compounds, electrovalent compounds generally have
-
Solution
Ionic compounds generally have high melting and boiling points because of the strong electrostatic force of attraction between oppositely charged ions.Consequently, a considerable amount of energy is required to overcome strong attractive.
Methanol and ethanol are miscible in water due to
-
Solution

Which of the following substances has the greatest ionic character ?
-
Solution

Identify the non polar molecule in the following compounds:
-
Solution
In H2, both atoms are identical, so the molecule is non polar.
Pauling’s electronegativity values for elements are useful in predicting :