The ions O2–, F–, Na+, Mg2+ and Al3+ are isoelectronic. Their ionic radii show
-
Solution
Amongst isoelectronic species, ionic radii of anion is more than that of cations. Further size of anion increase with increase in –ve change and size of cation decrease with increase in +ve charge. Hence ionic radii decreases from O2– to Al+++.
In hydrogen atom, energy of first excited state is –3.4 eV. Find out KE of the same orbit of Hydrogen atom
-
Solution

If a proton and α-particle are accelerated through the same potential difference, the ratio of de-Broglie wavelengths λp and λα is
-
Solution

The orbital diagram in which the Aufbau principle is violated is :
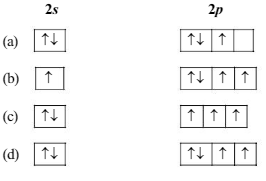
If the nitrogen atom has electronic configuration 1s7, it would have energy lower than that of the normal ground state configuration 1s22s22p3, because the electrons would be closer to the nucleus. Yet 1s7 is not observed because it violates.
-
Solution
As per Pauli exclusion principle "no two electrons in the same atom can have all the four quantum number sequal or an orbital cannot contain more than two electrons and it can accommodate two electrons only when their directions of spin are opposite".
The ratio of magnetic moments of Fe(III) and Co(II) is
-
Solution

The size of isoelectronic species C4–, N3– and Mg2+ is affected by
-
Solution
Because number of protons (nuclear charge) is different while number of electrons is same in isoelectronic species.
The number of nodal planes in a px orbital is
-
Solution

Given : The mass of electron is 9.11 × 10–31 kg Plank constant is 6.626 × 10–34Js,the uncertainty involved in the measurement of velocity within a distance of 0.1 Å is
-
Solution

If the shortest wavelength of the spectral line of H-atom in the Lyman series is X,then the longest wavelength of the line in Balmer series of Li2+ is
-
Solution






