Read the passage given below and answer the question that follow :J.W. Gibbs and H.Von Helmoltz had given two equations which are known as Gibbs-Helmholtz equation. One equation can be expressed in terms of change in free energy (ΔG) and enthalpy(ΔH) while other can be expressed in terms of change in internal energy (ΔE) and work function (ΔW)
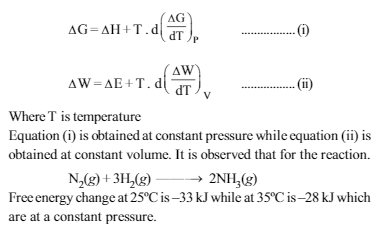
What would be the difference between enthalpy change at 25ºC and 35º C for a given reaction ?
-
Solution

A piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant temperature of 37.0ºC. As it does so, it absorbs 208 J of heat. The values of q and w for the process will be:(R = 8.314 J/mol K) (ln 7.5 = 2.01)
-
Solution
Process is isothermal reversible expansion,
hence ΔU = 0, therefore q = – W.Since q = +208 J, W= –208 J

-
Solution

The species which by definition has ZERO standard molar enthalpy of formation at 298 K is
-
Solution
The species in its elemental form has zero standard molar enthalpy of formation at 298 K. At 298K, Cl2 is gas while Br2 is liquid.
For the process H2O(l) (1 bar, 373 K) ⟶ H2O(g) (1 bar,373K), the correct set of thermodynamic parameters is
-
Solution
Since, liquid is passing into gaseous phase so entropy will increase and at 373 K during the phase transformation it remains at equilibrium. So, ΔG = 0.

-
Solution


-
Solution

For a particular reversible reaction at temperature T, ΔH and ΔS were found to be both +ve. If Te is the temperature at equilibrium, the reaction would be spontaneous when
-
Solution
At equilibrium ΔG = 0
Hence, ΔG= ΔH– TeΔS = 0
ΔH= TeΔS or Te = \(\frac{\Delta H}{\Delta s}\)
For a spontaneous reaction
ΔG must be negative which is possible only if ΔH– TΔS < 0 ∴ ΔH > TΔS
or T > \(\frac{\Delta H}{\Delta s}\);
Te < T

-
Solution


Assuming that water vapour is an ideal gas, the internal energy change (ΔU)when 1 mol of water is vapourised at 1 bar pressure and 100°C, (given : molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol-1 and R = 8.3 J mol-1 K –1) will be
-
Solution
Given ΔH = 41 kJ mol–1 = 41000 J mol-1
T = 100°C = 273 + 100 = 373 K
n =1
ΔU = ΔH – ΔnRT = 41000 – (1 × 8.314× 373)
=37898.88 J mol-1 ≃ 37.9 kJ mol-1




